
Organelles & small cells research innovation with Sophion's automated patch clamp
In automated patch clamp, patch holes with smaller diameter and thereby high resistance (3-7 MΩ) can be beneficial for forming giga-ohm seals with the cell membrane when working with organelles and small cells such as red blood cells, thus ensuring accurate measurements of ionic currents and membrane potentials.
High resistance small hole consumables cause less damage to the cell membrane, thus preserving cell integrity during the experiment recordings. The result is improved seal stability over time and reduced electrical noise, leading to clearer data. This is especially important when handling organelles and small cells, which are more delicate and susceptible to damage. These factors make high resistance consumables valuable in electrophysiological studies.
Exploring functions of organelles & small cells with Sophion APC platforms
The patch clamp technique has revolutionized our understanding of ion channel functions, providing detailed insights into the electrical properties of various cells. Automated patch clamp technology now enables the investigation of larger numbers of cells simultaneously. However, organelles and small cells patch clamping remains challenging due to technical limitations. Sophion platforms enable automated patch clamping with organelles like mitochondria and lysosomes and small cells like red blood cells. Our customized QPlate and QChip consumables are ideal for applications such as:
Organelles:
- Lysosomes
- Mitochondria
- Nucleus
Small cells:
- Primary/iPSC neurons
- Red blood cells
- T-cells
Investigating lysosomal ion channels with automated patch clamp
Lysosomes are organelles that contain enzymes for breaking down cellular waste and debris. Lysosomal patch clamp techniques enable the study of ion channels within lysosomes, providing valuable insights into their function and regulation. For example, the lysosomal cation channel TMEM175, which is associated with Parkinson’s disease, is a promising drug target. Automated patch clamp technology enhances these studies by allowing high throughput analysis and precise control over experimental conditions, facilitating a deeper understanding of lysosomal ion channels and their implications in disease.
Unveiling mitochondrial transporters with automated patch clamp
Mitochondria generate most of the chemical energy needed to power the cell’s biochemical reactions. They are surrounded by two membranes: an inner membrane and an outer membrane. Direct patch clamp recordings of mitoplasts (mitochondria devoid of the outer membrane) allow for the study of the biophysics of mitochondrial transporters in their native membrane. Automated patch clamp technology enhances these studies by enabling high throughput analysis and precise control over experimental conditions, providing deeper insights into mitochondrial function and its role in cellular energy production.
Advancing nuclear ion channels with automated patch clamp
The nucleus controls and regulates the activities of the cell and contains structures that carry hereditary information. Patch clamping on isolated nuclei is similar to patch clamping of isolated cells. Nuclear patch clamping is used to study ion channel activities within the nuclear envelope and intracellular membranes. By utilizing automated patch clamp technology, researchers can conduct high throughput analyses with precise control over experimental conditions. This approach provides valuable insights into the ion channel functions of the nucleus and its role in cellular regulation
Electrophysiology studies of primary neurons (and iPSC) with automated patch clamp
Electrophysiology studies are essential for understanding neuronal cells due to their excitable membranes and complex interaction. Automated patch clamp technology enhances our ability to explore brain function and unravel neurological disorders. High resistance consumables support the study of neurons with variable soma sizes ranging from 100 to 4 micrometres in diameter. Due to the fragile nature of neuronal preparations, these high resistance consumables also help preserve cell integrity during experimental recordings, ensuring more reliable and consistent results.
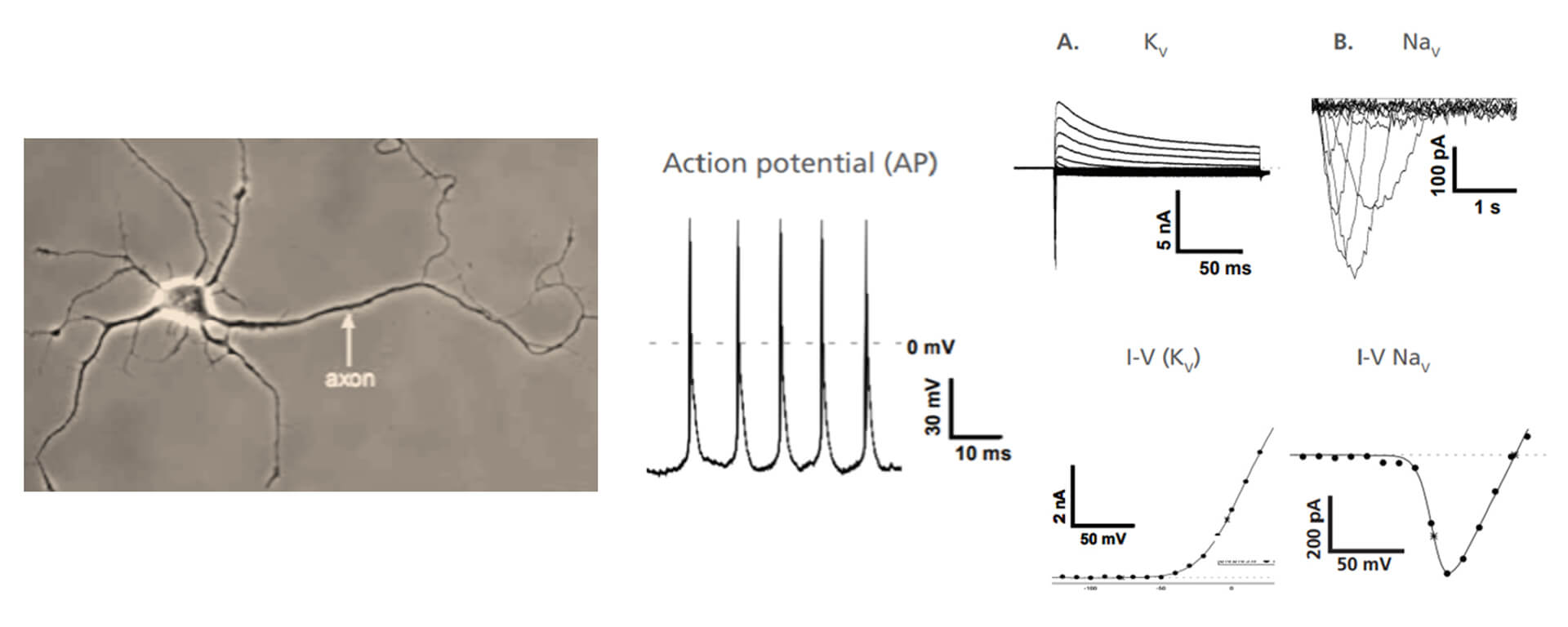
Isolated primary hippocampal neurons from mice were patched on the Qube 384 APC system. Using an optimized cell dissociation protocol to obtain healthy cell membranes for patch clamp in combination with high resistance QChips 384 custom, we obtained a whole-cell success rate of up to 65%. A. KV and B. NaV current recordings in primary hippocampal neurons and matching I-V curves. Action Potentials recorded in current clamp recording.
Enabling red blood cell research with automated patch clamp
Red blood cells, or erythrocytes, are the most abundant cell type in the blood and play a vital role in oxygen transport. These cells lack a nucleus and most organelles, posing challenges for automated patch clamp techniques. Their biconcave shape makes them flexible, allowing them to pass through narrow blood channels (capillaries) or patch holes if they are too large, which further complicates the process.
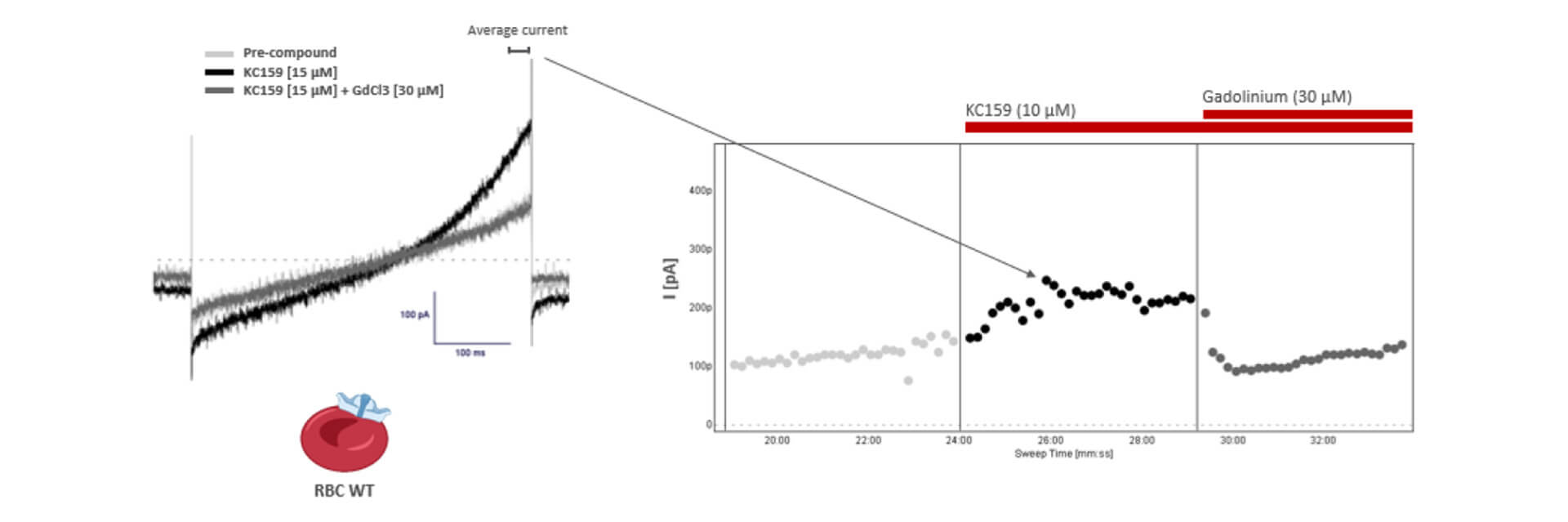
Automated high throughput patch clamp recordings on human RBCs expressing Piezo1 activity was assessed using a voltage ramp with activating (KC159) and inhibitory (GdCl3) compounds. These chemical modulators allowed us to explore the specific kinetics of Piezo1.
Exploring T-cell function with automated patch clamp technology
T-cells, or T lymphocytes, are a type of white blood cell that plays an essential role in the immune response. They are involved in recognizing and responding to pathogens. Automated patch clamp technology allows for the detailed study of T-cell activity, particularly in understanding the ion channel function of these cells. Automated patch clamp enables high throughput analysis, providing valuable insights into the electrophysiological properties of T-cells and their role in immune responses.
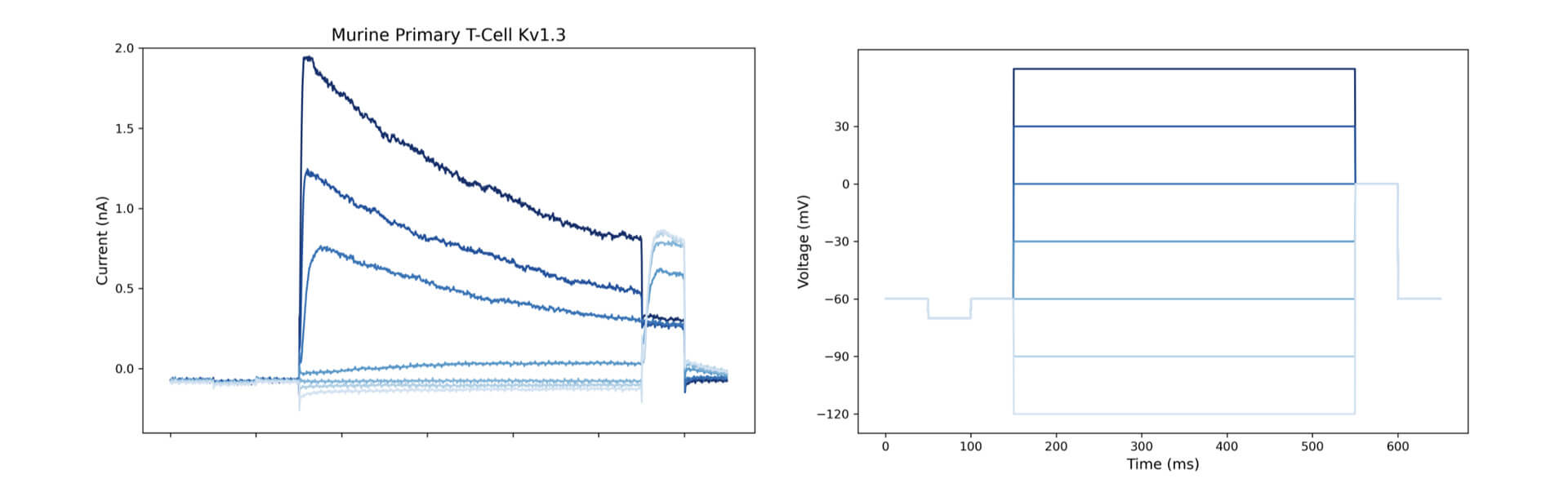
Automated high throughput patch clamp recordings on murine primary T-cell: A representative trace of a whole-cell patch-clamp Kv1.3 current and the activation by increasing voltage steps.
Click on the link below to view publications, videos, and application reports on small cells & organelles.
Get in Touch
We strive to provide the best for our customers, and we are always ready to help. Please let us know if you have a question for us.
