New Application Report is breaking barriers in lysosomal electrophysiology: automated assessment of lysosome channels
Lysosome ion channels are increasingly implicated in neurodegeneration and cellular clearance pathways. This latest application report demonstrates a robust, scalable automated patch clamp (APC) workflow for recording ion channel activity from enlarged lysosomes, validating TRPML1 pharmacology across Sophion’s QPatch 48 and Qube 384 platforms.
Lysosome channels: capture, seal quality and throughput
Using LYSO-Prep™ lysosomes enlarged with Vacuolin and single-hole, high-resistance consumables, we achieved consistent capture success rates of 69.9 ± 3.2% on the QPatch 48 and 74.6 ± 2.6% on the Qube 384. Stable seals were recorded without the need for fluoride-based enhancers, and smaller patch holes reduced ‘lysosome slipping’ through the recording site, improving resolution for currents <100 pA. These platform-level metrics indicate that APC can be used for medium to high throughput studies of lysosome ion channels.
Pharmacological validation of TRPML1 on APC systems
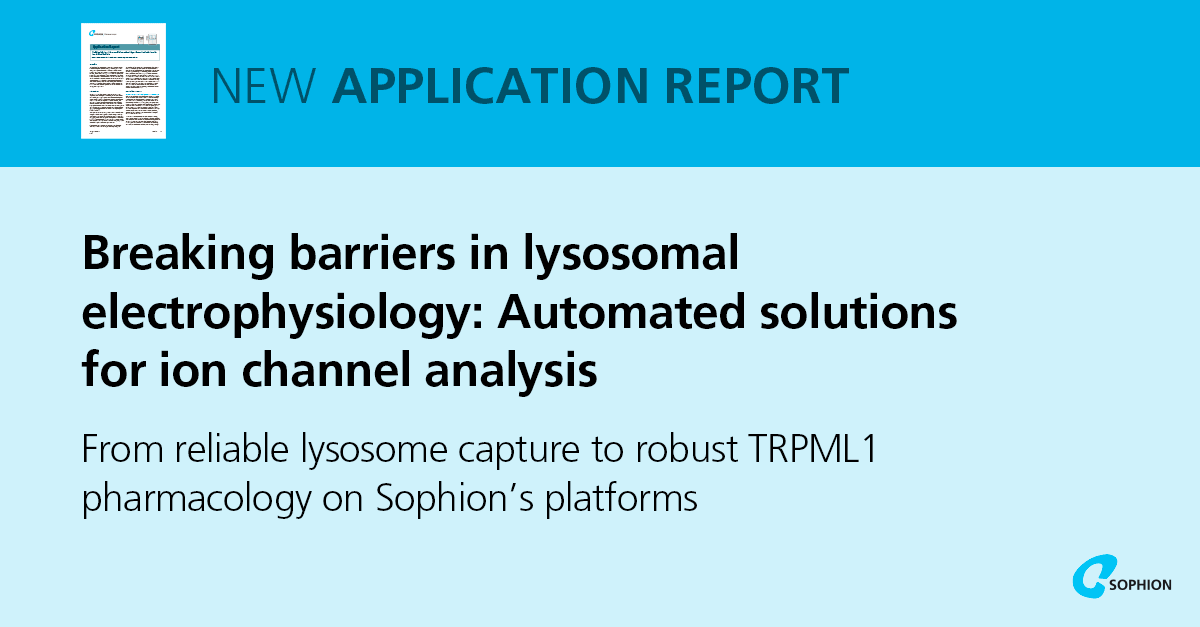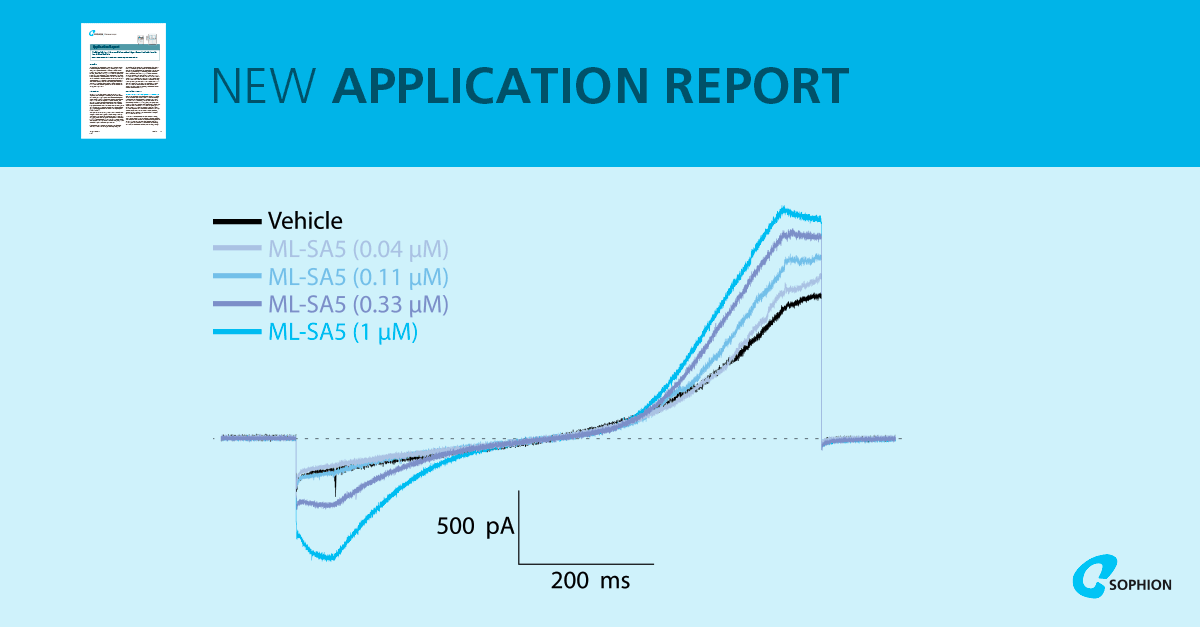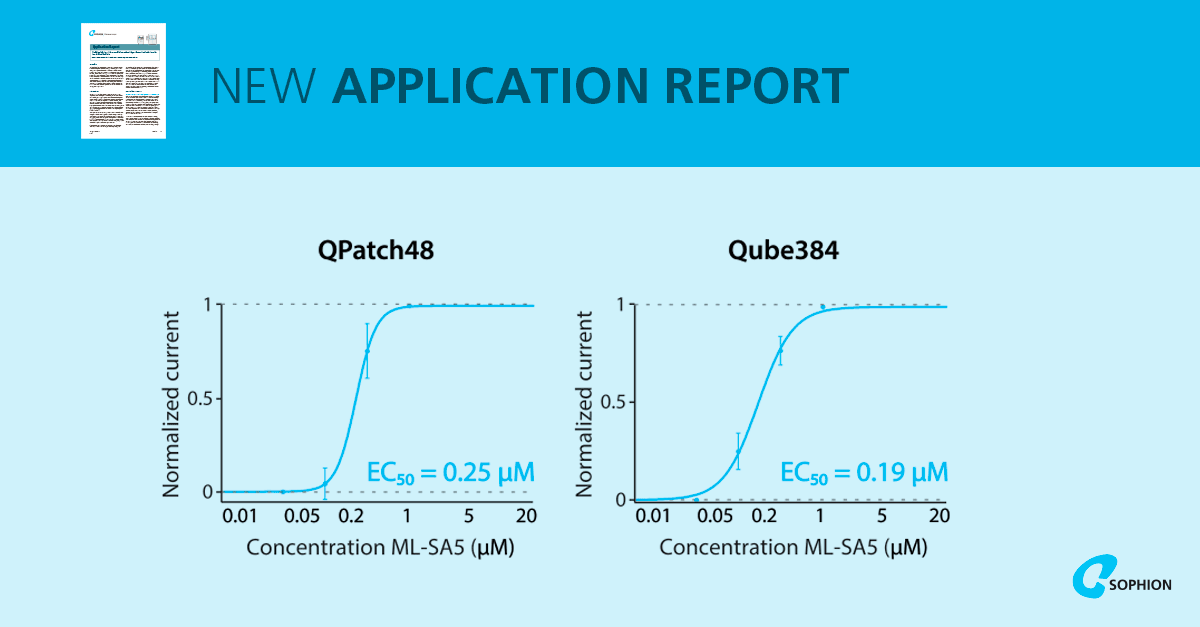
Functionally, TRPML1 overexpressing lysosomes responded to the selective agonist ML-SA5. Single concentration activation showed robust increases in both outward and inward currents, with ML-SA5-sensitive responses present in ~41–50% of successfully captured lysosomes across the two platforms. TRPML1 knockout lysosomes did not respond to ML-SA5, confirming specificity. Cumulative concentration–response experiments produced EC50 values of 0.25 µM on QPatch and 0.19 µM on Qube, consistent with published whole-endolysosomal recordings. Importantly, a concentration experiment performed at 35°C produced a comparable success rate to 22°C, supporting work at near-physiological temperature.
Enabling reliable, high throughput lysosomal electrophysiology
The work shows that reliable lysosome capture, stable seals and reproducible concentration–response data can be obtained at room temperature and at near-physiological temperature:
- Automated patch clamp with high-resistance (HiR) single-hole consumables enables reliable capture of enlarged lysosomes and stable seals suitable for electrophysiology studies.
- TRPML1 currents activated by ML-SA5 were reproducible on both QPatch 48 and Qube 384, with EC50 values matching literature benchmarks.
- Specificity was confirmed by absence of ML-SA5 responses in TRPML1 knockout lysosomes.
- The assay performs at RT and at 35°C, allowing physiologically relevant pharmacology without loss of throughput.
- Using HiR consumables reduced the potential for ‘lysosome slipping’ through the recording site and improved detection of small currents, making the method suitable for screening and detailed mechanistic studies.
This application report reflects the excellent collaboration with Oria Bioscience
