Shocking discoveries in iPSC neuron electrical maturity
Human-induced pluripotent stem cell (hiPSC)‑derived neurons are increasingly used to model neurological diseases, but variability in electrophysiological maturity and the low throughput of manual patch‑clamp techniques have hampered robust functional studies.
In collaboration with FUJIFILM Cellular Dynamics, in Sophion’s latest application report, we demonstrate how automated patch clamp (APC) can be used to both characterize ion channel function and classify neuronal maturity. Thereby, enabling more precise definition and selection of cells for disease modeling.
Key findings include:
- High-throughput APC assay with robust success rates:
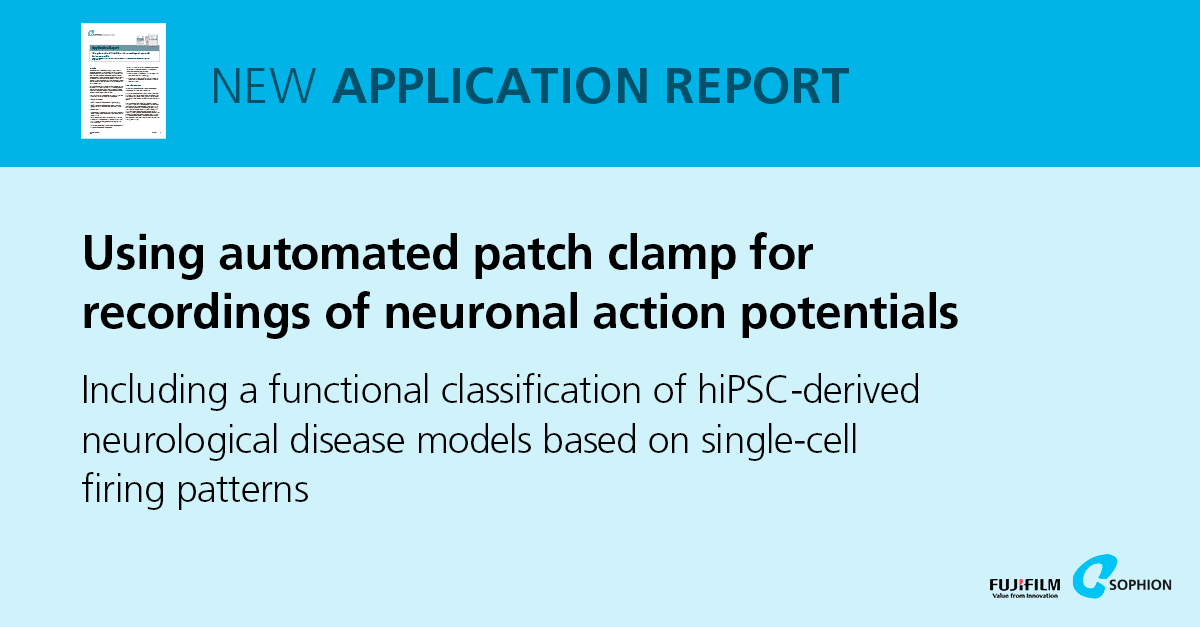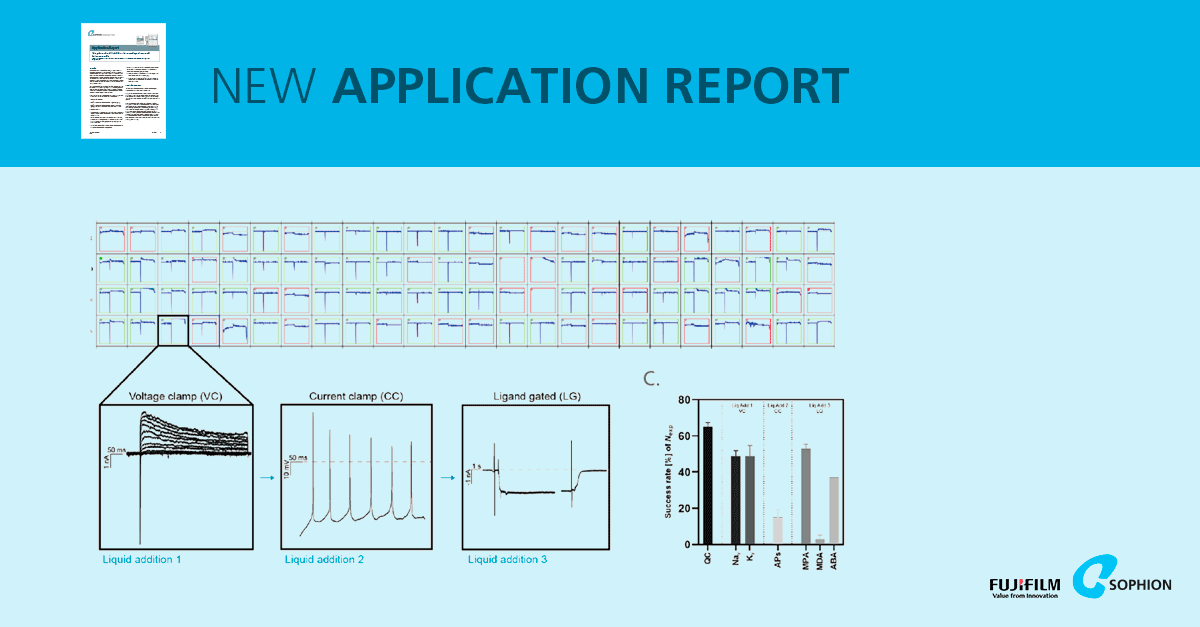
An APC workflow was established on Sophion Qube and QPatch platforms, optimizing cell dissociation to achieve ~60 % whole‑cell recording success across 384‑site consumables. Sequential protocols recorded voltage‑gated Na⁺/K⁺ currents, current‑clamp action potentials, and ligand‑gated AMPA, NMDA and GABA currents in the same cells. - Comprehensive electrophysiological profiling of three hiPSC‑derived lines:
iCell® GlutaNeurons, WT excitatory neurons, and GRN R493X heterozygous knockout excitatory neurons were profiled. Across DIV (days in vitro), all lines yielded measurable NaV, KV, AP and ligand‑gated currents, illustrating maturation‑dependent increases in functional channel expression. - Functional classification into four maturity types:
Based on Bardy et al. (2016), neurons were sorted into T1–T4 based on the number of action potentials fired during a current‑step protocol: T1 (no AP), T2 (1 AP), T3 (2 APs), T4 (>2 APs). The proportion of highly mature T4 neurons increased with DIV and was consistent across both APC systems. - Correlation of electrophysiological parameters with maturity:
More mature T4 neurons showed significantly higher NaV current density, slightly reduced steady‑state KV currents, increased AMPA‑mediated currents, larger capacitance (cell size), and more hyperpolarized resting membrane potentials – hallmarks of functional maturation - Impaired maturation in GRN frontotemporal dementia model:
Compared to isogenic WT controls, GRN R493X neurons exhibited a lower proportion of T4 cells that failed to increase over time. T4 GRN neurons had reduced NaV current density, lower AP peak potentials and amplitudes, and slower maximum depolarization rates, indicating compromised excitability. - Network‑level validation via MEA and neurite assays:
Multi‑electrode array recordings at DIV 21 showed GRN cultures with longer time‑to‑burst peaks, while live‑cell imaging over DIV 0–14 revealed reduced neurite outgrowth in GRN versus WT neurons, corroborating single‑cell APC findings. - Culture purity and identity confirmed:
Immunostaining of iCell GlutaNeurons for βIII‑tubulin and PSD95 demonstrated healthy neuronal networks; flow cytometry indicated ≥ 90 % neuronal purity and ~ 75 % glutamatergic identity by single‑cell gene expression. - APC‑based workflow enables precise disease phenotyping:
By combining high‑throughput APC with functional classification, researchers can select electrophysiologically comparable neurons, improving the fidelity of hiPSC‑based disease models and facilitating drug discovery efforts.
This study underscores the power of APC platforms to standardize functional assessments, reduce variability, and accelerate insights into neurodegenerative disease mechanisms. The application report and related research was developed by Sophion Bioscience in collaboration with FUJIFILM Cellular Dynamics.